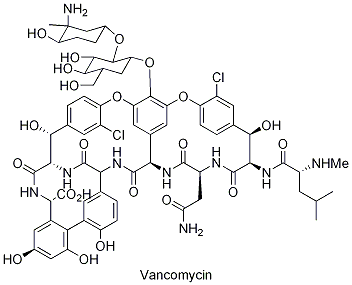
Vancomycin
Basic Information
Vancomycin is a front line glycopeptide antibiotic made by Amycolatopsis orientalis (previously Streptomyces orientalis). It is used to disrupt cross-peptide linking in gram-positive bacteria and most importantly, to combat methicillin resistant Staphylococcus aureus (MRSA). This antibiotic can only be injected intravenously, though it can be taken orally to treat pseudomembranous colitis resulting from Clostridium Difficile, a complication caused by other antibiotic use. Vancomycin is formed via the non-ribosomal peptide synthetase pathway, also known as NRPS.
top
History
A missionary first collected Vancomysin in a soil sample from the Borneo jungles, and the antibiotic was extracted by the Eli Lilly chemist EC Kornfeld.
Medical Applications
Total Synthesis
The total synthesis of Vancomycin was completed by K.C. Nicolau.
top
Biosynthesis
Vancomycin is a glycopeptide composed of a heptapeptide backbone via the non-ribosomal peptide synthase pathway (NRPS) and modified by glycosylation. The peptide backbone contains seven amino acids (three non-proteogenomic amino acids, four natural) linked together by the NRPS pathway. After the heptapeptide backbone is completed, A methyl transferase adds a methyl group to the amine end. Then, the peptide backbone’s three rings are cyclized by oxidative phenol coupling through the heme enzymes Oxy A, Oxy B, and Oxy C. The enzymes Oxy A, Oxy B, and Oxy C catalyzing the phenol coupling reactions belong to the cytochrome P450 family of hemoproteins. Two glycosyl transferases add the carbohydrates vancosamine (non-natural) and glucose (natural) last.
The three non-proteogenomic amino acids are dihydroxy phenyl glycine (dpg), β-hydroxychloro tyrosine (β-OH Tyr), and hydroxyphenyl glycine (hpg). The β -hydroxychlorotyrosine and 4-hydroxyphenylglycine residues come from L-tyrosine, while the 3,5-dihydroxyphenylglycine is made from acetate. The biosyntheses of hydroxyphenyl glycine and β-hydroxychloro tyrosine are shown in figure 3 below. Thirty genes code for vancomycin within the biosynthetic operon – four NRPS, three oxidative P450, one halogenation, four Hpg, and four others.
top
Figure 1:

NRPS Pathway for Vancomycin Biosynthesis

top
Biosynthesis of the non-proteogenomic amino acids (figure 3)

top
References
[1] Woithe, K., Geib, N., Zerbe, K., Li, D.B., Heck, M., Fournier-Rousset, S., Meyer, O., Vitali, F., Matoba, N., Abou-Hadeed, K., and Robinson, J.A. “Oxidative Phenol Coupling Reactions Catalyzed by OxyB: A Cytochrome P450 from the Vancomycin Producing Organism. Implications for Vancomycin Biosynthesis” J. Am. Chem. Soc., 129, 21, 6887 - 6895, 2007.
[2] Derwick, Paul M. Medicinal Natural Products: A Biosynthetic Approach. John Wiley & Sons Ltd, The Atrium, Southern Gate, Chichester, West Sussex PO19 8SQ, England. Pp. 426-427, 2001.
[3] “Vancomycin” http://en.wikipedia.org/wiki/Vancomycin.
[4] Burkart, Mike. “Vancomycin Lecture” Chem 157. University of California, San Diego, 5 June 2007.
top
Comments (0)
You don't have permission to comment on this page.